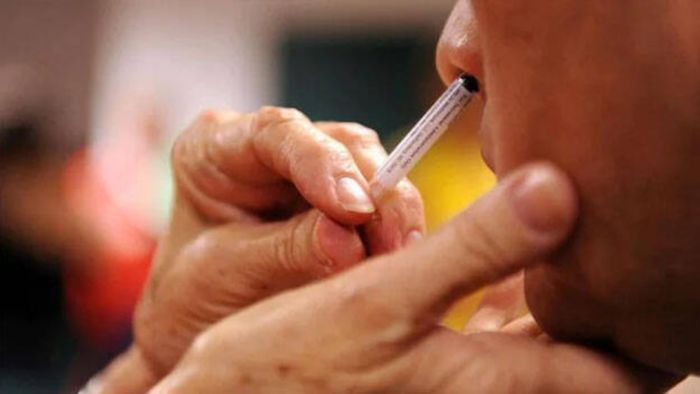
Government of India approves nasal vaccine for COVID-19, to be available on CoWIN app
Notably, the intranasal Covid vaccine will be first available in private hospitals and is said to be included in the Covid Vaccination program effective December 22.
- Dec 23, 2022,
- Updated Dec 23, 2022, 12:45 PM IST
The Union Minister Dr Mansukh Mandaviya announced that Bharat Biotech nasal vaccine has been approved by the central government to be introduced as a heterologous booster.
Notably, the intranasal Covid vaccine will be first available in private hospitals and is said to be included in the Covid Vaccination program effective December 22.
It is to be mentioned here that the Nasal vaccine in India is the first in the rest of the world to introduce a needless jab.
The jab will be made available to those above 18 years, however, the price of the vaccine will be decided soon.
Also Read: Omicron Sub-Variant BF.7 in India: Transmission Rate, Symptoms and Precautions
Meanwhile, currently, Bharat Biotech’s Covaxin, Serum Institute’s Covishield and Covovax, Russian Sputnik V and Biological E Ltd’s Corbevax are listed in the CoWin portal.
Earlier on December 22, Prime Minister Narendra Modi held a high-level meeting to review the situation related to COVID-19 in the country.
Union Health Minister Mansukh Mandaviya, Home Minister Amit Shah, Aviation Minister Jyotiraditya Scindia and top officials attended the virtual meeting.
Ahead of the meeting, in a statement in Lok Sabha, Minister Mandaviya asked states to remain alert and create awareness about wearing face masks and using hand sanitisers, especially in view of upcoming festivals and new year celebrations.