IIT-Guwahati Scientists formulate efficient catalytic systems for Biofuel and Lactic Acid Production
- Nov 16, 2020,
- Updated Nov 16, 2020, 12:48 AM IST
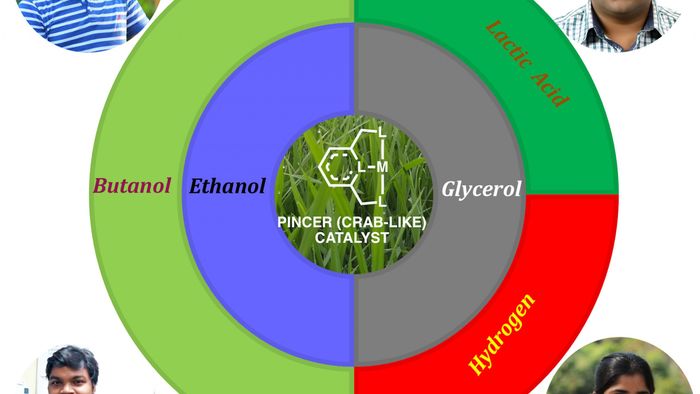
GUWAHATI: A research team led by IIT Guwahati Professors have formulated efficient “pincer” catalytic systems that transform industrial/biomass wastes into valuable chemicals. Tiny amounts of these “pincer catalysts” repeatedly convert large amounts of industrial waste such as glycerol into lactic acid and hydrogen. Such catalysts also efficiently convert bioethanol, a low-energy-density fuel into high-energy-density butanol.
The conversion of valuable intermediates such as glycerol and ethanol, produced during the processing of biomass, into industrially useful chemicals has elicited much interest worldwide. Glycerol, for example, which is a by-product in biodiesel production, can be transformed into lactic acid and hydrogen, the former used extensively in food, pharmaceutical, cosmetic and polymer industries, and the latter in the energy sector. Likewise, ethanol obtained from biomass can be converted into high quality fuel. While bioethanol has lower energy density than gasoline and corrodes engine parts when used directly, it can be transformed into higher energy butanol that is immiscible in water and noncorrosive in nature. The conversion of glycerol and ethanol into such useful products hinges on the development of efficient catalysts that can bring about these transformations.
Also Read: Assam: Last rites of martyred jawan Haradhan Roy performed today
Dr. Akshai Kumar and Dr. Hemant Kumar Srivastava work towards the development of catalysts that can bring about such industrially important transformations. They have recently developed efficient ‘pincer catalysts’ that selectively convert glycerol to lactic acid and bio-ethanol to butanol.
“Pincer catalysts are complex molecules in which, an organic moiety holds on tightly to a metal core, much like the claws of a crab”, explains Dr. Akshai Kumar, adding that such an arrangement not only confers stability to the catalyst, but also selectivity to bring about the intended transformations.
The research team rationally designed and tested a large library of “pincer catalysts” to be used for these transformations. The experiments were carried out under environmentally benign conditions without the use of hazardous reagents and solvents. The most efficient “pincer catalyst” was found to be one that had least crowding around the metal centre. Such an arrangement enabled easy removal of hydrogen from the starting materials, glycerol and ethanol, and their selective conversion into lactic acid and butanol, respectively.
The results of the experiments have been validated by theoretical studies.“Our computational studies have attributed the unprecedented activity of the pincer catalysts to the minimal crowding present at the metal centre and have enabled good understanding of the electronic and steric (crowding) factors that control reactivity,” says Dr. Srivastava
“This catalyst is active over several thousand cycles without loss in efficiency,” says Dr. Akshai Kumar. He adds that the generation of hydrogen in the conversion of glycerol to lactic acid is a bonus, given its enormous demand in energy applications.
The findings have recently appeared in the Royal Society of Chemistry journals, Chemical Communications and Catalysis Science & Technology. The research team plans to take these bench-scale reactions to pilot-plant scale and ultimately to the commercial level with industrial collaboration. The research team believes that the work will have a global impact on the commercial production of lactic acid/biofuels and their multi-billion-dollar market worldwide.
To support our brand of fearless and investigative journalism, support us HERE.
Download:
The Inside Northeast app HERE for News, Views, and Reviews from Northeast India.
Do keep following us for news on-the-go. We deliver the Northeast.